Why Validation with LandingLens?
Companies regulated by the FDA or other regulatory bodies face a unique set of challenges when attempting to bring products to market quickly and safely. LandingLens offers a computer vision platform with validation packages that makes it simpler for companies to keep up with compliance requirements, reduce validation-related time and cost, and make upgrades swiftly so customers’ needs are always met. With this reliable platform, you can now have the peace of mind of knowing your products work the way they are intended.
LandingLens simplifies software validation
- A platform with standardized workflow streamlines validation process and documentation
- Advance release notification
- Validation document and process support
- Access our validation experts
Using the latest AI-based technologies will
not only speed up the time needed for the products to come to the market, but will also improve the quality of products and the overall safety of the production process, and provide better utilization of available resources along
with being cost-effective, thereby increasing
the importance of automation”.
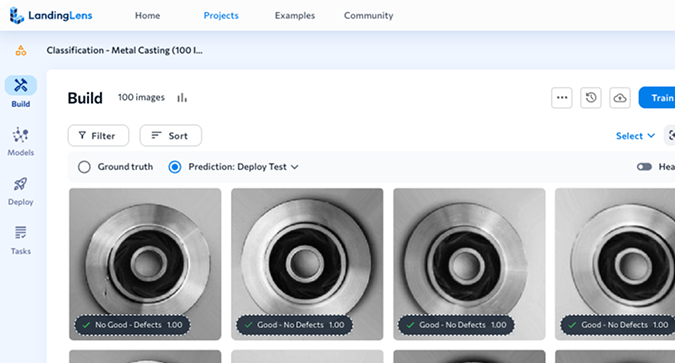
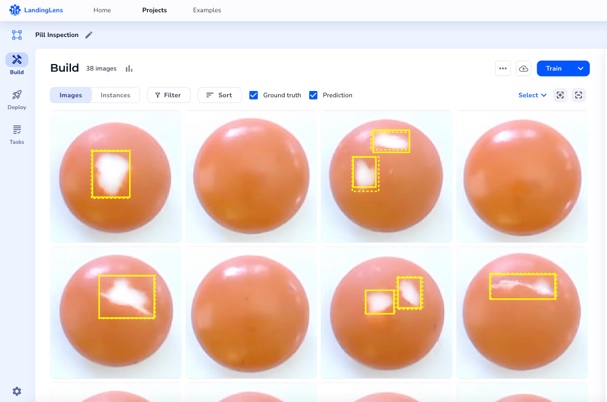
Benefits
LandingLens, an industry leading computer vision cloud platform, helps improve inspection accuracy and reduce false positives. The end-to-end platform standardizes deep learning solutions that reduce development time and scale projects easily to multiple facilities across the globe.
Take Product Quality to the Next Level
Inspection accuracy is critical, especially in regulated industries. Whether you’re developing the newest life-saving drugs or revolutionary medical devices, every product must be of the highest quality. LandingLens can catch any defects in the production line, leaving no room for error. With LandingLens, you can focus on what matters most — creating groundbreaking innovations that will affect people’s lives.


Accelerate Innovation
With LandingLens, validation becomes a seamless part of the development process. By leveraging pre-built validation documentation provided by LandingLens, teams can focus solely on the execution of the validation and greatly speed up the entire process. This enables teams to focus on their core competencies, without being slowed down by the hassles of validation. In this way, teams can move faster, make better decisions, and bring higher-quality products to market more quickly.
Cut Validation Times by up to 80%
In today’s fast-paced world, speedy development and execution are crucial for companies to stay ahead of the competition. Validation with LandingLens reduces the time and cost associated with the validation of evolving software. You can streamline your process and optimize your documentation so that your operations are running at peak performance. By leveraging the knowledge and expertise of our validation partner, you can be confident that your company is on track to success.

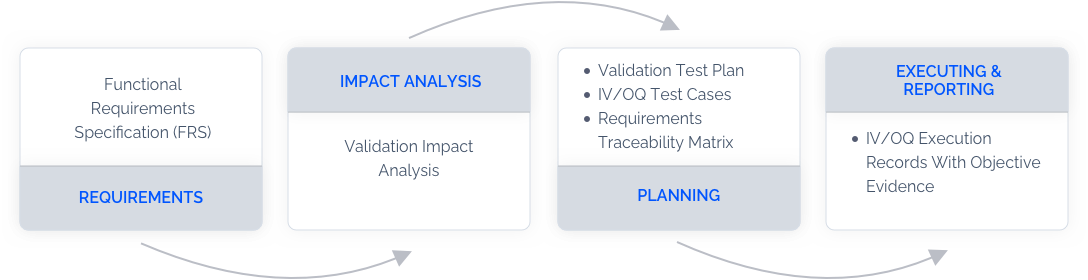
How Does Validation with LandingLens Work?

Access Release Notes
in Advance
Access to release notes 2 months prior to release so that your team can effectively allocate resources to access impacts and learn how to leverage the benefits of upcoming features.
Access Validation Documentations
Access validation documentations with requirements, impact analysis, protocols, and execution records. Our validation experts can assist documentation and validation process.
Validate Your Solutions
in Staging Environment
Test and validate your solutions in a staging environment prior to the new release being pushed to the production environment.

SOC 2 Type II compliance
LandingAI is compliant with applicable SOC 2 controls and maintains a SOC 2 report. Developed by the American Institute of CPAs (AICPA), SOC 2 is the name of a report that can be provided by a certified auditing body to a service organization. SOC 2 defines controls around 5 target categories called trust service criteria: security, availability, processing integrity, confidentiality, and privacy. SOC2 report also meets requirements for NIST 800-53 and ISO 27001Resources

WEBINAR
Accelerate FDA Compliance for Your Computer Vision Systems

TECH BRIEF
LandingLens Simplifies FDA Software Compliance

WHITE PAPER