AI Visual Inspection for Medical Devices and Pharma Compliance
Prevent defects, ensure quality, and meet strict standards with AI-powered computer vision. Our visual inspection solutions help achieve compliance with pharma and medical device regulations while reducing risks, recalls, and costly delays.
“The [LandingLens] platform allows us to generate accurate and consistent datasets that we can iterate over time to continuously improve our existing AI systems.”
— Ligand Pharmaceuticals
Applications for AI in Pharmaceuticals
AI-powered computer vision enhances quality control across the medical device and pharmaceutical industry. These solutions support visual inspection workflows and apply AI to medical devices and pharma manufacturing to deliver greater accuracy, compliance, regulatory adherence, and production efficiency.
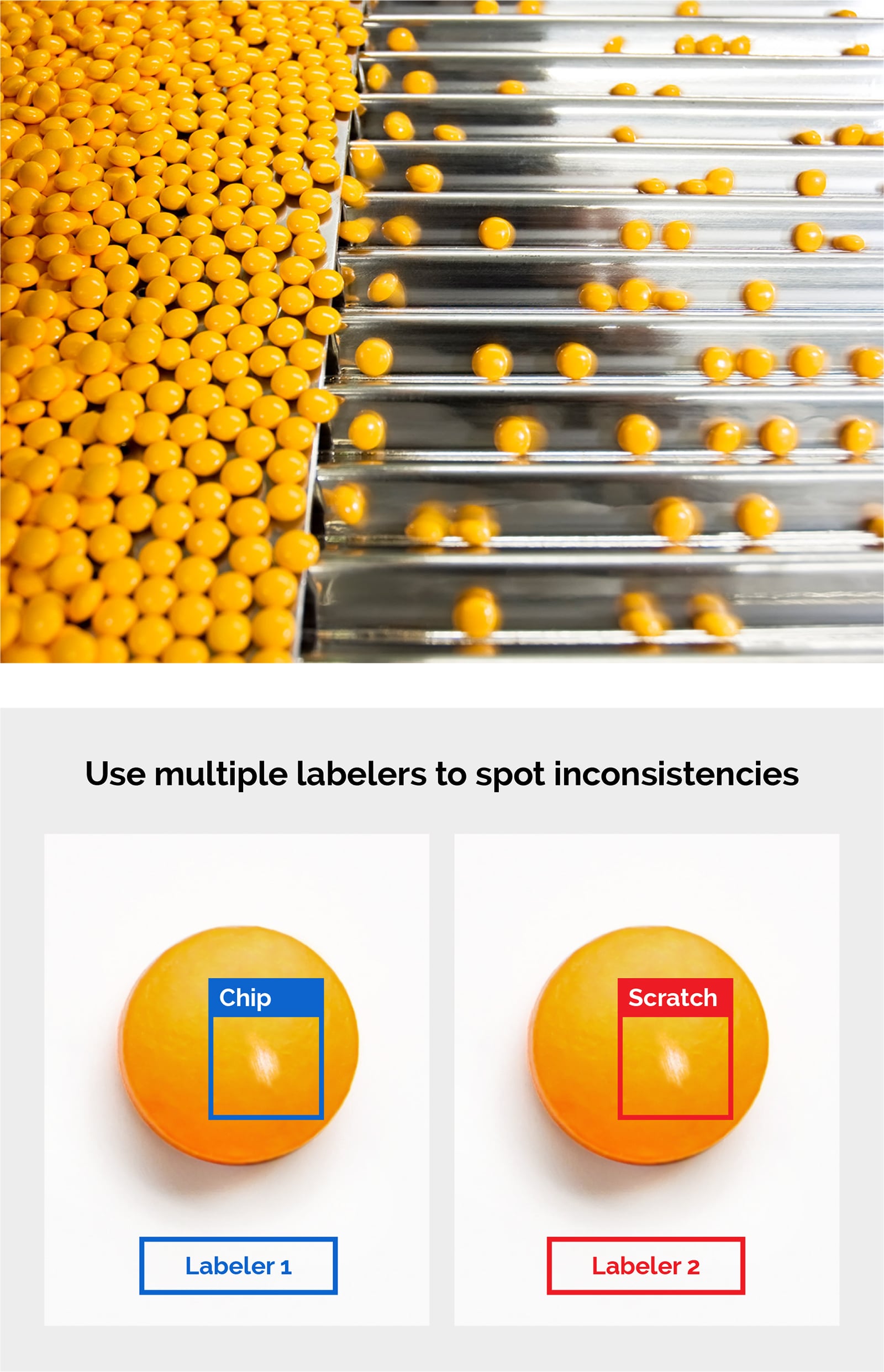
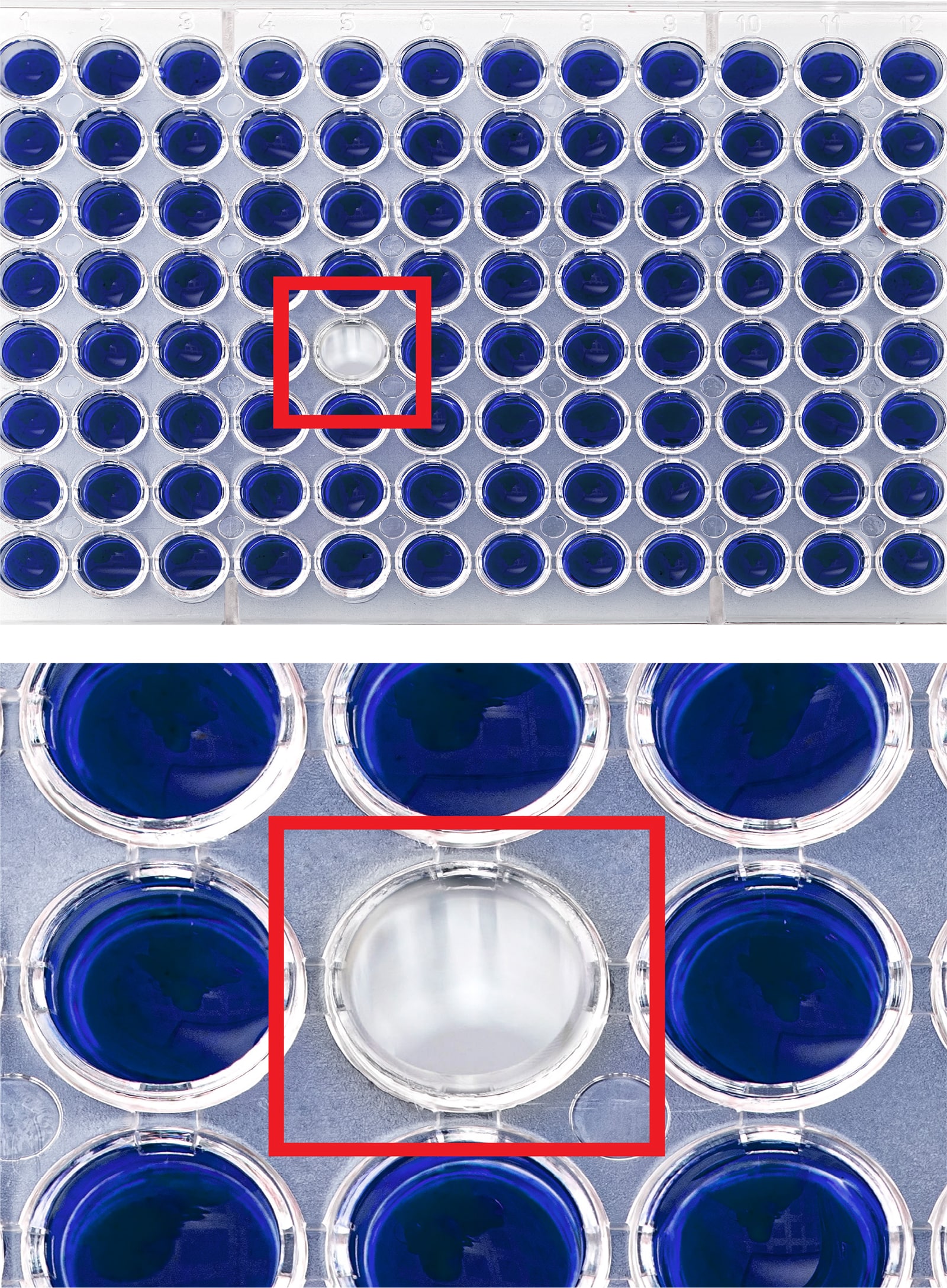
- Pill InspectionPrior to being placed into bottles and other containers, tablets and capsules must be inspected for defects and abnormalities such as cross-contamination, broken pills, and incorrect pills. Machine vision systems have long helped with such visual inspection tasks, but deep learning software can improve inspection accuracy of such systems.LandingLens powers AI-driven visual inspection in pharma manufacturing workflows by training on images of both defective and non-defective material, enabling faster and more accurate quality control.
- Vial CountingMachine vision technology can automate vial counting, saving time and reducing the risks of human error, such as misplaced or missing vials and costly recalls. While machine vision systems already offer visual counting capabilities, deep learning software like LandingLens adds a vital layer of accuracy by verifying counts and detecting defects before products leave the facility. This verification is a key part of pharma regulatory compliance, where precision is essential.
- Medical Device Inspection (Nano-Scale)While several inspection tasks in medical and pharmaceutical settings can be done manually, the inspection of nano-scale components is not one of them. Manufacturers must deploy imaging technology to detect defects and abnormalities, often down to the micron level. Manual inspection with a microscope involves multiple steps and can be prone to human errors and inconsistencies. Deep learning software, like LandingLens, can automatically process microscopy images and classify defects or irregularities, making AI-powered visual inspection a valuable tool in medical device manufacturing and high-precision pharmaceutical industry applications.
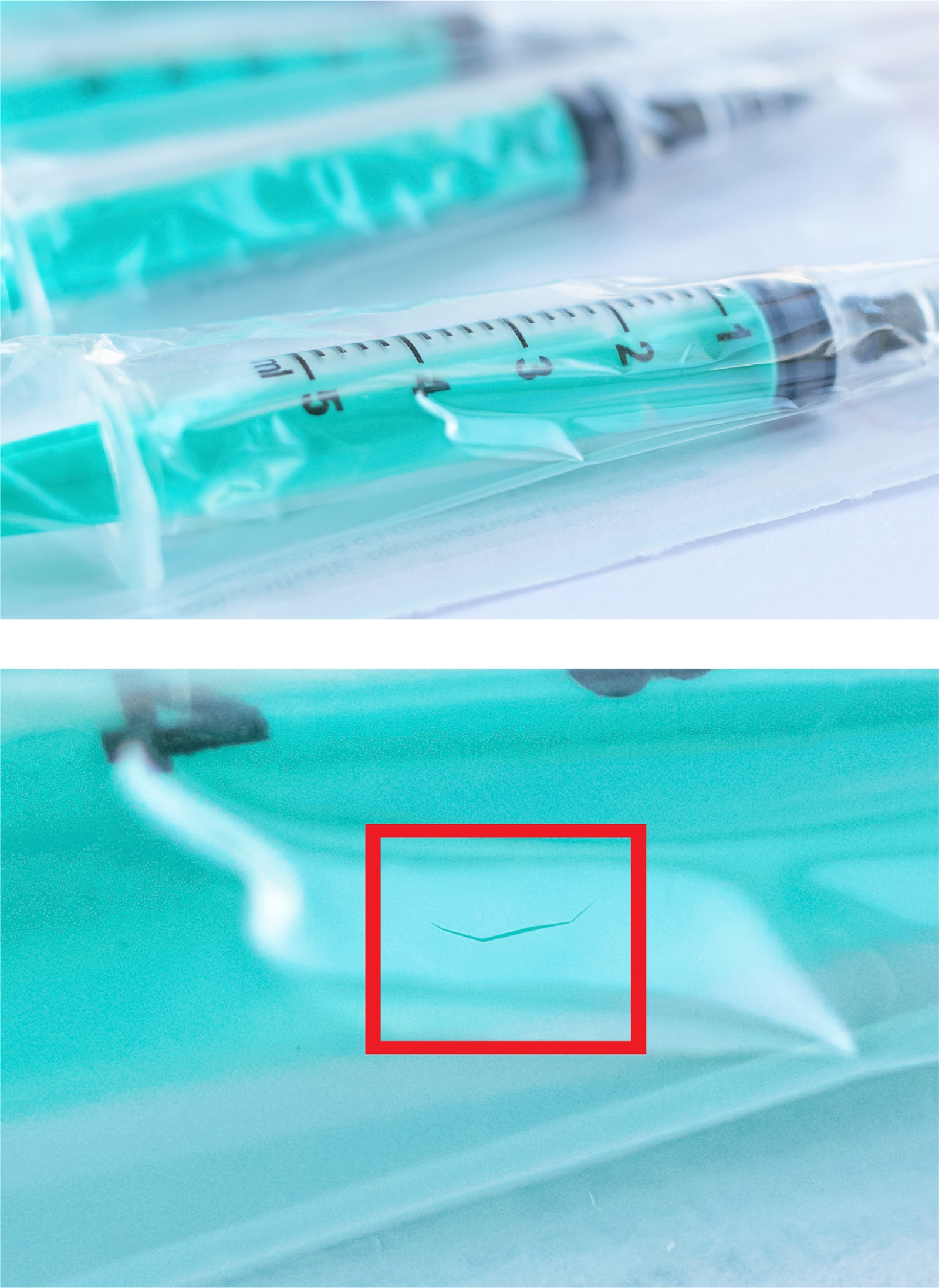
- Vial Contamination InspectionVials and syringes must be thoroughly inspected before shipping, as contaminants can mean the difference between life and death. They must also be checked for cracks and other defects to prevent safety issues, recalls, lost revenue, and damage to customer trust. Machine vision technology can assist with vial and syringe inspection, but rules-based algorithms often struggle with the range of defect types and false positives caused by transparency and reflections. LandingLens enables teams to build reliable AI models, automating processes once considered impossible and helping ensure quality and compliance in pharma manufacturing.
- Medical Device Seal InspectionWhether it’s a surgical tool, ventilator, or heart valve, medical devices must be sealed into sterile packaging before being shipped off to the customer. Failure to do so presents risks to patient safety, company reputation, and revenue at risk. While traditional machine vision can automate inspection, LandingLens excels in scenarios that challenge rules-based methods, such as detecting contamination or improper seals. These capabilities are critical for ensuring quality and meeting medical device compliance requirements.
- Medical Device PCB InspectionPrinted circuit boards in medical devices require thorough inspection, but performing mode and effects analysis for each wafer, pin, joint, and environmental change is complex. Rules-based machine vision often struggles to detect certain defects, such as tilts, burns, foreign material, voids, and chips. LandingLens enables AI-powered visual inspection, allowing manufacturers to detect and classify these issues accurately while supporting the medical device industry’s regulatory compliance requirements.
- Final Assembly VerificationBefore products ship, manufacturers must verify that medical devices and other products are correctly assembled and ready for use. This includes locating, inspecting, and identifying components. While machine vision systems often perform these tasks, factors such as lighting changes, random product orientation, and multiple defect types can make manual inspection seem necessary. AI-powered deep learning delivers a consistent, automated approach to assembly verification, ensuring high quality and compliance in both pharmaceutical and medical device manufacturing.
- Biomedical ApplicationsDeep learning has diverse applications in biomedical research, from identifying the number, location, and type of cells in images to aiding in disease detection, drug discovery, clinical trials, and genomics pattern recognition. These capabilities demonstrate how AI for medical devices and the pharmaceutical industry can accelerate innovation while ensuring regulatory readiness.



The Benefits of LandingLens AI for the Pharmaceutical Industry
LandingLens is an industry-first AI solution with a data-centric approach. Our visual inspection system for pharmaceutical manufacturing leverages artificial intelligence to enhance accuracy by minimizing false positives. The end-to-end platform standardizes deep learning solutions that reduce development time and scale projects easily to multiple facilities across the globe. Launch your first model free, upgrade anytime.
Meet Strict Regulations, Keep Compliant
Medical and pharmaceutical companies must follow strict regulations from agencies such as the Food and Drug Administration. Maintaining product quality is key to complying with these regulations. LandingLens deep learning software can help companies stay compliant by improving inspection accuracy and catching defects and irregularities early in the process. Manufacturers in this industry are also subject to process validations which help demonstrate that the manufacturing process will consistently produce conforming products. LandingLens can help support the validation process by maintaining clean, thorough data and results.


Achieve Zero Product Escapes
There’s no room for error when it comes to manufacturing environments, and this is especially true for medical and pharmaceutical manufacturing. Companies need machine vision and deep learning software systems to prevent defective products from leaving the factory floor. This is crucial for avoiding corrective actions, increasing productivity, delivering quality products, and maintaining solid customer relationships.