Interview with Quinn Killough by LandingAI
Table of content
- How will AI help make better medical devices and drugs, and bring them to market faster?
- Why is Validation with LandingLens needed?
- What’s the goal of validation?
- Who can use LandingLens Validation Package?
- Must every change to a piece of software be validated, per FDA regulations?
- What happens if companies don’t validate?
- Who is using LandingLens and what result did it have?
- LandingAI has a partnership with Verista for the validation. What does Verista do?
- What if there is a cybersecurity issue? Will LandingLens users have to wait six months for a fix?
- How is LandingLens different from other AI and computer vision platforms?
LandingLens is a computer vision platform that makes it easy to acquire and label data, ensure data quality, and train AI models. When used in manufacturing, LandingLens helps companies spot and eradicate manufacturing defects and allows for continuous improvement through machine learning to improve the reliability, speed, and accuracy of manufacturing processes.
LandingLens improves efficiency by replacing highly manual inspection with automated inspection, and by quickly training AI models to accurately spot issues so they can be dealt with.
LandingAI recently introduced validation support for LandingLens, which is a solution that caters to the specific requirements in highly regulated industries. We sat down with Quinn Killough, Sales Director at LandingAI, to talk about validating software to conform to FDA compliance requirements and how LendingLens can address these challenges.
How will AI help make better medical devices and drugs, and bring them to market faster?
Computer vision, which enables software to analyze images, will be used in every industry to make products and services better, more quickly. In the life sciences industries, we anticipate that AI and computer vision will be game-changing technologies that will have as many uses as imaging allows. Life sciences, because of regulation, the need for safety and validation, have been slower than many other industries to adopt these technologies. Validation with LandingLens will help change that.
Validation with
LandingLens
Why is Validation with LandingLens needed?
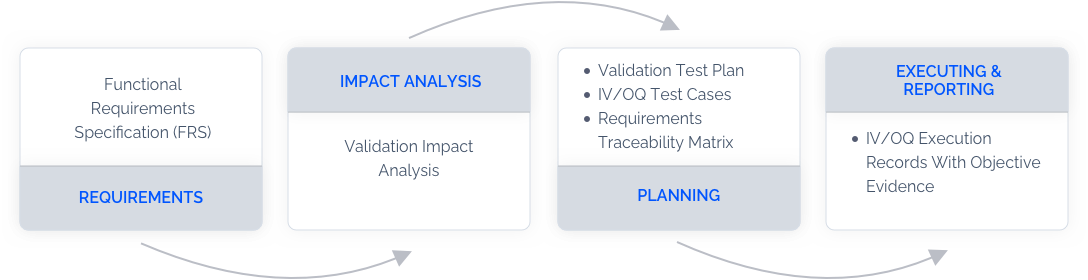
The FDA regulates medical devices and drug development. In the interest of safety, it mandates that all software tools be validated, meaning they be checked and tested to ensure that they will perform a certain way all the time. This helps maintain the safe production and delivery of medical devices and drugs.
As companies embrace the benefits of using cloud-based software, keeping up with validation becomes problematic. LandingLens offers the best of both worlds — a cloud-based computer vision platform that is on a controlled release cycle to manage compliance.
In today’s world, software changes too fast for companies to continually validate. As a result they might:
- Miss out on innovations in tools because they don’t or can’t regularly validate their tools.
- Put software on-premise and then freeze it and miss out on the benefits of the cloud.
- Continually validate, which is time-consuming and costly.
LandingAI will roll out updates to Validation with LandingLens every six months. This schedule enables companies to use the latest advancements in computer vision and have ample time to validate the software updates.. Companies get notified of updates at five months, can test the next month, and then roll the new version out in month six.
This preset schedule helps companies in regulated industries plan and deploy innovative tools more readily.
What’s the goal of validation?
To ensure that medical device and drug manufacturers make high quality products and that their manufacturing systems and software will do the same things, over and over, reliably.
Who can use LandingLens Validation Package?
LandingLens Validation Package is great for any company in a highly-regulated industry that needs to validate tools to pass regulatory muster. In the US alone, FDA-regulated companies, which includes more than 20,000 drug products and 6700 different medical device product categories. The FDA also classifies more than 8500 companies as significantly regulated organizations.
Must every change to a piece of software be validated, per FDA regulations?
No. But every change must be assessed as to whether or not it needs to be validated because it is a regulated function. Companies need a change control process for this and must be in compliance.
What happens if companies don’t validate?
The FDA inspects these companies and will cite them for being out of compliance. This can be costly in terms of remediation and reputation. Safety and trust are foundational for medical devices and drug makers.
Who is using LandingLens and what result did it have?
One of top 5 global medical device makers deploy LandingLens. They reduced the time it took for the initial deployment validation from 3 months to 1 to 2 weeks. LandingLens is also being used by a wide range of companies in the manufacturing, biomedical and other industries.
LandingAI has a partnership with Verista for the validation. What does Verista do?
Verista is a leader in manufacturing equipment validation, vision automation, and cloud-based solution platform validation. That’s a rare and unique find in the marketplace. Verista supports the validation to build test plans and documentation that is required for the validation process. Verista offers a tailored approach to documentation and reporting that allow you to achieve compliance with ease.
What if there is a cybersecurity issue? Will LandingLens users have to wait six months for a fix?
No, if an emergency fix is needed, such as in the case of a cybersecurity issue, LandingAI will make the change immediately.
How is LandingLens different from other AI and computer vision platforms?
First, the platform is so intuitive that even none data scientists and AI engineers can use it and quickly put it to work. Second, LandingLens’s Data-Centric AI makes models work even with small datasets by ensuring data quality. Having a limited number of images is very common for many companies in many industries, and LandingLens still lets them unlock the massive value of AI. Last but not least, the cloud-based platform makes scaling projects simple from a single production line to worldwide operations.